
To calculate the degree of unsaturation, enter the values of a, b, c, d and e and press "calculate".Īlternatively, to calculate the number of hydrogen atoms, enter values for the degree of unsaturation, a, c, d and e and press "calculate". This calculator can be used to obtain this number from the information which is easier to read from the structure: the number of carbon and heteroatoms and the " degree of unsaturation".įor a compound with formula C aH bN cO dX e where X is F, Cl, Br or I, the degree of unsaturation is given by:ĭegree of unsaturation = 1/2 (2 + 2a - b + c - e) However as the hydrogen atoms which are bonded to carbon are not shown on stick structures, it is common for mistakes to be made in working out the number of hydrogen atoms from the stick structure. Why calculate the number of hydrogen atoms? Stick structure are an extremely convenient for representing organic structures. This calculator calculates the " degree of unsaturation" directly from the formula. As well as dictating the number and type of atoms in a compound, the molecular formula controls the number and types of bonds that are present. Why calculate the degree of unsaturation?Ī common problem in organic chemistry is trying to work out possible structural formulas for a compound having a particular molecular formula. If the total degree of unsaturation is calculated from the molecular formula, it can greatly help establish possible structures. Triple bonds count as two degrees of unsaturation. Each ring and double bond counts as one degree of unsaturation. It is the chemical formula which represents the actual number of atoms of each element present in one molecule of the compound. Every ring and multiple bond in a compound is a 'degree of unsaturation'. Note: You can use other related calculator Chemical Equation Balancer.Saturated organic molecules possess only multiple bonds and no rings.

Therefore the polyatomic ion for the above positive and negative ions is Lithium Chloride. The hydroxide cation (OH-) and the phosphate cation (PO3− 4) are both polyatomic ions.īarium has a +2 charge and hydroxide has a -1 charge, thereforeġ Ba 2+ ion is required to balance 2 OH- ions Ammonium has a +1 charge and phosphate has a -3 charge, thereforeģ NH 4 + ions are required to balance 1 PO 4 3- ion Potassium has a +1 charge and sulfate has a -2 charge, thereforeĢ K + ions are required to balance 1 SO 4 2- ionĬalculate the polyatomic ion for the given positive and negative ions. Polyatomic ions consist of multiple atoms that combine with other ions to form an ionic compound. In an ionic compound, electrons are transferred to atoms and molecules imparting a charge on both the donor and recipient of the electron.Ī polyatomic ion considers two or more atoms at a time as constituents. The balance chemical equations calculator balance each half of the reaction separately. Please do not change the formula while balancing it. Add the appropriate ratio that comes before the chemical formula.
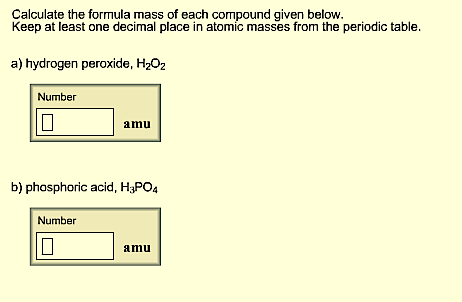
Phosphate - PO 43- Cation and Anion calculatorĪ polyatomic ion, also known as a molecular ion, is a charged species (ion) composed of two or more atoms covalently bonded or of a metal complex that can be considered as acting as a single unit in the context of acid and base chemistry or in the formation of salts.īy putting the positive ion and the negative ion in the respective boxes, you can find the scientific name of that polyatomic by using ion calculator. The chemical equation must have the same number of atoms for each element on both sides of the equation. This will give you the mass percent of the chemical. Write down the information you have for the charges of the component ions and balance them to answer the problem.īicarbonate (or hydrogen carbonate) - HCO 3- Divide the mass of the chemical by the total mass of the compound and multiply by 100. When there are two or more polyatomic ions in a formula, enclose the polyatomic ion in parentheses. When you write the formula for an ionic compound, remember that the positive ion is always listed first.


 0 kommentar(er)
0 kommentar(er)
